$ 0.001 3.62%
Molecule (MOL) Rank 4646
| Mkt.Cap | $ 0.00000000 | Volume 24H | 0.00000000MOL |
| Market share | 0% | Total Supply | 40 BMOL |
| Proof type | N/A | Open | $ 0.0013 |
| Low | $ 0.0013 | High | $ 0.0014 |
Science: Is air a compound or a mixture?
Other liquids with lower surface tension would have a higher tendency to "rip", forming vacuum or air pockets and rendering the xylem vessel inoperative. Whether something is a molecule or not depends on the type of bond that is formed when its atoms join together. In general, electrons can be shared between atoms (a molecular bond) or electrons can be completely removed from one atom and given to another (an ionic bond). Complex molecules are made up of carbon bonded with other elements, especially oxygen, hydrogen and nitrogen.
Molecules can be represented with a chemical formula which shows the types of atoms in the molecule, and, uses subscripts, ti show how many of each type of atom is present. Amacromoleculeis a very large molecule, such as protein, commonly created by the polymerization of smaller subunits (monomers). They are typically composed of thousands of atoms or more. The most common macromolecules in biochemistry are biopolymers (nucleic acids, proteins, carbohydrates and polyphenols) and large non-polymeric molecules (such aslipids (fats) and macrocycles).

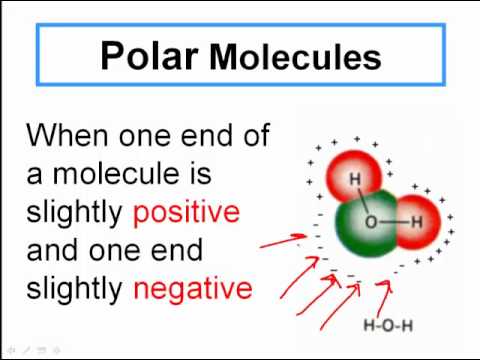
An important feature of the water molecule is its polar nature. The water molecule forms an angle, with hydrogen atoms at the tips and oxygen at the vertex. Since oxygen has a higher electronegativity than hydrogen, the side of the molecule with the oxygen atom has a partial negative charge. A molecule with such a charge difference is called a dipole.
Humans require water that does not contain too much salt or other impurities. Common impurities include chemicals or harmful bacteria. Some solutes are acceptable and even desirable for perceived taste enhancement. Water that is suitable for drinking is termed potable water. The solid state of water is known as ice; the gaseous state is known as water vapor (or steam).
You can pour liquid acid on a solid and watch the solid dissolve. That process is a chemical change because molecular bonds are being created and destroyed. Geologists pour acids on rocks to test for certain compounds. The study of molecules by molecular physics and theoretical chemistry is largely based on quantum mechanics and is essential for the understanding of the chemical bond. The simplest of molecules is the hydrogen molecule-ion, H2+, and the simplest of all the chemical bonds is the one-electron bond.
It contains one magnesium (Mg) and two chlorine (Cl) atoms. Methane (CH4) is made up of one carbon (C) and four hydrogen (H) atoms.
compliance as a service (CaaS)
“There is not a single entity that can challenge the loading capacity of our PG5,” he says. They then performed several similar cycles, adding sub-branches to each existing branch, to build tree-like structures. In total, the whole synthesis required 170,000 bond formations, Schlüter says. Water is made up of hydrogen and oxygen, as the others have said.
We need it for all parts of our body to function. Can you guess how we classify water, which plays such a large role in living organisms? It's a molecular substance, which is a substance with two or more atoms, the smallest units of matter, joined together by a covalent bond.

Hydrogen gas (H2) is a molecule, but not a compound because it is made of only one element. Water (H2O) can be called a molecule or a compound because it is made of hydrogen (H) and oxygen (O) atoms. When atoms come together to form ionic or covalent bonds, they form stable associations that change the properties of either atom alone.

Sodium chloride (NaCl) is a classic example of an ionic compound, or compound formed by ionic bonds. Water (H2O) is often called a molecular compound, but is also known as a covalent compound because it is a compound formed by covalent bonds. Although less common, some biology textbooks refer to molecular compounds as molecules, and do not include ionic compounds in the term molecule. All molecules can be written as a list of the atoms forming them.
Water is considered a simple molecule made of just a few atoms. Complex molecule have many atoms joined together in some cases a repeating pattern.
Science: Is air a compound or a mixture?
- Water is also a molecule because it is made from atoms that have been chemically combined.
- As this example also points out, the atoms in a molecular formula are not always written in order of their bonds.
- Stereoisomers, a particular type of isomer, may have very similar physico-chemical properties and at the same time different biochemical activities.
- As you stir, atoms from the water in the tea are attracted to the atoms in the sugar.
- Synthetic macromolecules include common plastics and synthetic fibers as well as experimental materials such ascarbon nanotubes.

A pure substance is composed of molecules with the same average geometrical structure. The chemical formula and the structure of a molecule are the two important factors that determine its properties, particularly its reactivity.
Related QuestionsMore Answers Below
What type of molecule is water?
Water is a chemical compound and polar molecule, which is liquid at standard temperature and pressure. It has the chemical formula H2O, meaning that one molecule of water is composed of two hydrogen atoms and one oxygen atom. Water is found almost everywhere on earth and is required by all known life.
Water boils at 100 °C and methane boils at -161.5 °C. Low molar mass molecules, such as H2 and CH4, tend to form gases. Whether a substance made of molecules is solid, liquid, or gas at room temperature is determined by the molecules' molar mass and the strength of electrostatic attraction between molecules. The terms molecule, compound, and atom can be confusing!
Can we see atom?
An atom is one of the basic units of matter. Everything around us is made up of atoms. An atom is a million times smaller than the thickest human hair. The diameter of an atom ranges from about 0.1 to 0.5 nanometers (1 × 10−10 m to 5 × 10−10 m).
While many molecular substances are soluble in organic, or carbon-based, solvents, they are generally insoluble in water. Instead of dissolving, the molecules might form hydrogen bonds with the water molecules or react in some other way with the water. When oxygen bonds to itself (e.g., O2, O3) or to another element (e.g., carbon dioxide or CO2), molecules are formed. Chemical changes in compounds happen when chemical bonds are created or destroyed. Forces act on the bonds between atoms, changing the molecular structure of a substance.
It contains three atoms and is called a triatomic molecule. they want to get to a full outer shell; hydrogen has 1 already and needs 1 more, oxygen has 6 already and needs 2 more.
Who Is the Father of Chemistry?

Molecules are made up of atoms that are held together by chemical bonds. These bonds form as a result of the sharing or exchange of electrons among atoms. The atoms of certain elements readily bond with other atoms to form molecules. Examples of such elements are oxygen and chlorine.
How water molecule is formed?
Water is a polar molecule A water molecule is formed when two atoms of hydrogen bond covalently with an atom of oxygen. In a covalent bond electrons are shared between atoms. In water the sharing is not equal.
In a liquid, the molecules can move freely among each other, in a sort of slithering fashion. In a gas, the density of molecules is generally less than in a liquid or solid of the same chemical compound, and they move even more freely than in a liquid.
About 70% of the Earth's surface is covered by water. Water is known to exist, in ice form, on several other bodies in the solar system and beyond, and proof that it exists (or did exist) in liquid form anywhere besides Earth would be strong evidence of extraterrestrial life. Molecules can vary greatly in size and complexity. Some molecules consist of two atoms of the same element.
What are the 3 types of molecules?
Water is a dipolar molecule because each atom has a dipole, or partial charge. Oxygen is more electronegative than hydrogen, and thus pulls the shared electrons in the covalent bond closer towards its nucleus. This gives oxygen a partial negative charge and hydrogen a partial positive charge.
of a molecule shows the symbol for every atom, and it shows every bond. SEE ALSO -Molecular Modeling for K You will be able to interactively study molecules online using Jsmol. A polymer is formed when smaller molecules join together to form one large molecule.

Oxygen in the atmosphere is a molecule because it contains molecular bonds. It is not a compound because it is made from atoms of only one element - oxygen. This type of molecule is called a diatomic molecule, a molecule made from two atoms of the same type. Thestructural formulaof a chemical compound is a graphic representation of the molecular structure, showing how the atoms are arranged.








